40+ years of innovation
INNOVATIVE
For more than 40 years D.O.R.C. Dutch Ophthalmic Research Center (International) B.V. has worked intensively with leading top surgeons. Therefore at D.O.R.C. we know exactly which requirements instruments and equipment must satisfy. Our knowledge, combined with a unique mix of talent for the development of new ideas, high design quality and a strong product philosophy, results in highly innovative products.
PERSONAL
The clients, surgeons from all four corners of the earth are D.O.R.C.'s focal point. Our approach is both personal and professional. We do everything possible to realize innovative and affordable solutions. And all of our efforts are subject to high ethical and moral standards. That is why, in close collaboration with the physicians, we continuously subject our procedures and products to rigorous evaluations.
WORLDWIDE
D.O.R.C. Dutch Ophthalmic Research Center (International) B.V. exports its instruments and equipment to more than eighty countries worldwide and has its own sales and marketing organisations in Austria, Belgium, Brazil, China, Finland, France, Germany, India, Italy, the Netherlands, Norway, Spain, Sweden, UK, USA.
80+
countries where D.O.R.C. products are sold
Over a period of 40 years, D.O.R.C. has grown into a successful international business. An intensive cooperation with ophthalmic surgeons from throughout the world together with D.O.R.C. passion for innovation continues to result in high quality products.
-
1983
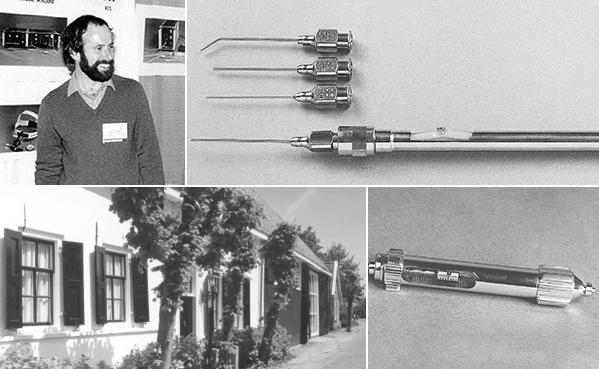
As a qualified instrument maker Ger Vijfvinkel became fascinated by ophthalmic surgery. To develop new instruments he observed operations, talked to surgeons and helped them to find solutions for their problems. D.O.R.C. was officially founded in 1983. -
1988

The developments in these innovative instruments did not remain unnoticed and international demand grew. D.O.R.C gradually developed into a professional company. -
1992

A number of specialized products were developed at the end of the 1980s. For example, the four port cannula system and the Harmony system. Since 1992 D.O.R.C. has had its own sales team and it now markets and sells all of its products. -
1999

By the end of the 1990s D.O.R.C. had become a dynamic organisation. The research and development team grew in terms of both expertise and size. The sales growth has led to subsidiaries in the USA, France, UK, Austria, Germany and Scandinavia. -
2023

Yet despite these changes D.O.R.C. still has the same identity: external focus, clear thinking, inclusiveness & expertise. Thanks to these core values our products have been and will be making a vital contribution to the development of ophthalmic surgery.
CERTIFICATES
ISO-13485-2016
CMDCAS
Medical Devices Directive 93/42/E
FDA
Quality Assurance
D.O.R.C. is committed to quality and the continuing improvement of product and Quality Management System to meet customer and global regulatory requirements. D.O.R.C. has the aspiration to increase the image of D.O.R.C. as a high quality supplier of ophthalmic surgical instruments, equipment, liquids and accessories.
The quality policy includes achieving customer satisfaction through good insight in our customer's requirements as well as the evaluation of information relating to the customer perceptions as to whether D.O.R.C. has met the customer requirements.
D.O.R.C. complies with international regulatory requirement and is certified for
- ISO-13485-2016
- CMDCAS
- Medical Devices Directive 93/42/EEC
- and is FDA registered.